Quality Control
Basic Policy
The MEDIPAL Group considers it its social mission to comply with pharmaceutical laws and regulations beginning with the Pharmaceutical and Medical Device Act, and to distribute safe, reliable pharmaceuticals and medical equipment, etc. To accomplish this mission, we strive to implement thorough quality control processes, from pharmaceutical product receipt to delivery to medical institutions.
Quality Control Systems
The Corporate Reliability Department supervises the status of logistics quality control at the four prescription pharmaceutical wholesalers. At ALC, in particular, which is a logistics site, the Corporate Reliability Department is promoting efforts aimed at consolidating methods based on the GDP* guidelines and at improving ongoing logistics quality. Furthermore, the department is also working to outfit FLCs and branches throughout Japan with organizational systems based on the guidelines and the necessary capital investments, while implementing educational activities for employees responsible for product management and distribution. In addition, to increase reliability in logistics quality, the department is further enhancing our pharmaceutical distribution practices by strengthening cooperation not only with relevant internal departments but also with pharmaceutical companies, distributors, and other external partners.
* GDP (Good Distribution Practice) : Appropriate procedures for ensuring proper distribution of pharmaceuticals and standards for ensuring maintaining the integrity of pharmaceuticals through the proper management of distribution channels in the form of purchase, storage, and supply, and preventing the entry of counterfeit drugs into regular distribution channels.
- MEDICEO CORPORATION,
- EVERLTH Co., Ltd.,
- ATOL CO., LTD.,
- SPLine Corporation
Maintaining Quality
For quality control in the storage and distribution of pharmaceuticals, medical equipment, and other products, the MEDIPAL Group strives to build and operate appropriate systems by creating manuals on logistics operations, supervising pharmacist operations, etc., based on ordinances issued by the Ministry of Health, Labour and Welfare, and on JGSP1 and JGSP2008.
The Group also formulates manuals for quality control and standard operating procedures (SOPs) in accordance with the globally harmonized JGSP/GDP,*1 revised to reflect PIC/S*2 GDP, and with GDP guidelines issued by the Ministry of Health, Labour and Welfare. In addition, the MEDIPAL Group works to enhance management systems, provide opportunities for suggesting improvements at GDP review meetings, and implement educational activities.
The Group is now providing training on quality control manuals and SOPs to the logistics departments of the four prescription pharmaceutical wholesalers as well as to all ALCs. In addition, we carry out regular quality reviews to promote and enhance the quality of GDP activities. In FY2022, we also expanded our GDP activities, which had previously focused on distribution centers, to FLC and branches. At EVERLTH Co., Ltd. and ATOL CO., LTD., we confirmed consolidation of such activities in FY2023. We are also advancing the development of GDP activities per area from FY2024 at MEDICEO CORPORATION.
*1 JGSP (Japanese Good Supplying Practice: Practices regarding quality control and safety management in the supply of pharmaceuticals): Industry practices defined by The Federation of Japan Pharmaceutical Wholesalers Association in order to protect the safety of products and prevent their degradation due to temperature, humidity, sunlight, etc., during storage, shipping, and transport. JGSP applies to prescription pharmaceuticals, while JGSP2008 applies to over-the-counter pharmaceuticals.
*2 PIC/S (Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme)
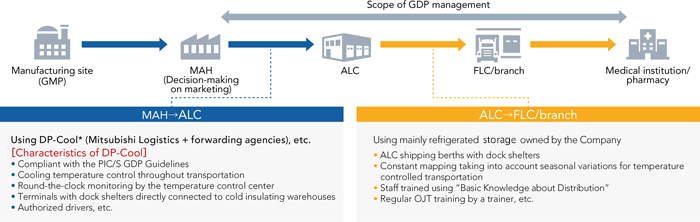
* DP-Cool: Refrigerated transportation service for pharmaceuticals compliant with the PIC/S GDP guidelines
- MEDICEO CORPORATION,
- EVERLTH Co., Ltd.,
- ATOL CO., LTD.,
- SPLine Corporation
Education and Training
The MEDIPAL Group provides systematic and ongoing training to maintain the integrity of pharmaceuticals, etc. The training is based on the annual education and training plan formulated at the beginning of the fiscal year and covers quality control manuals and SOPs for employees and personnel in charge of merchandise management and distribution. The training combines multiple methodologies including online training sessions, classroom study, and on-the-job training (OJT) to meet various roles and levels of mastery.
- Introductory Education and Training
Scope: New assignees/personnel
Contents:Learn the basic procedures and acquire knowledge for performing work in each department
Hours:Approx. 5 hours - Regular Education and Training
Scope: Employees and personnel responsible for product management and distribution
Contents:Acquiring knowledge of GDP and knowledge related to quality control matters such as drug regulatory affairs and supply of pharmaceutical products handled by the Company
Hours: 10+ hours
- MEDICEO CORPORATION,
- EVERLTH Co., Ltd.,
- ATOL CO., LTD.,
- SPLine Corporation
