Activities Report / InformationREPORT & INFORMATION
2024.10.01
Medical and disease
information
- #Real-World Data
- #RWD
- #Evidence
What is the relationship between Real-World Data and Healthcare?
The terms Real-World Data (RWD) and Real-World Evidence (RWE) have become common in healthcare. Although these words may be unfamiliar to patients and their families, RWD is essential in developing better treatments, especially drugs for rare diseases. This article will provide a clear explanation of these terms.
1. What is Real-World Data?
According to the U.S. Food and Drug Administration (FDA), RWD and RWE are defined as follows1).
Real-World Data (RWD):
RWD are data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources.
Real-World Evidence (RWE):
RWE is the clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of RWD.
Sources of information for RWD include data from electronic health records (EHRs), medical claims data, disease treatment data registries, health surveys, and health-related apps. With advances in information technology, the sources of information have become more diverse, allowing for a broader range of information to be collected.
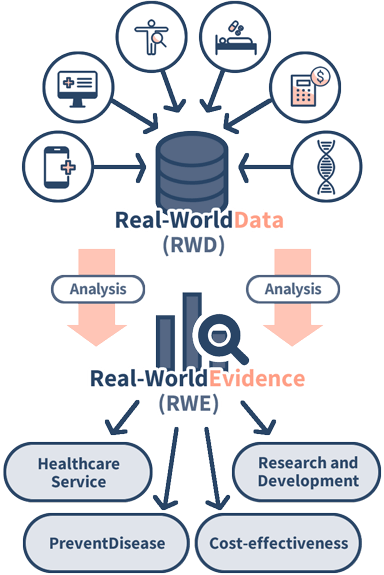
While clinical trials collect information within a set of conditions, RWD collect data from various situations in the actual setting. Although each piece of data may seem small on its own, the large amount of data and the diversity of backgrounds make the data highly valuable depending on the purpose of use.
The use of personal data in RWD naturally raises privacy concerns. When RWD are collected and used, it is done with the individual’s consent.
For example, when physicians or pharmaceutical companies directly collect patient information for research and development, they must always obtain the patient’s consent. In addition, information that could lead to the identification of individuals is removed or processed in databases available to companies and organizations, making it impossible to know to whom the data belongs.
2. What is the Real-World Data used for?
According to the FDA’s draft revised guidelines for using RWE for medical devices, the following are situations where RWD can be utilized3). While these are examples of utilization-focused medical devices, it is considered that they could be utilized in similar situations for drug products.
- To generate hypotheses to be tested in a clinical study;
- As a historical control, an informative prior in a Bayesian analysis of a clinical trial, or as one source of data in a hierarchical model or a hybrid data synthesis;
- As a concurrent control group or as a mechanism for collecting data to support marketing authorization when a registry, EHR, claims data or some other systematic data collection mechanism exists;
- As a mechanism for re-training artificial intelligence/machine learning-enabled medical devices;
- To generate evidence to identify, demonstrate, or support the clinical validity of a biomarker or clinical outcome assessment;
- To generate (primary) clinical evidence to support marketing authorization (e.g., HDE, PMA, 510(k) or De Novo request);
- To generate evidence directly by the subject device to provide new information on safety or effectiveness;
- To generate evidence to support a determination on whether the subject device meets the statutory criteria for a CLIA waiver (e.g., CW and Duals);
- To generate evidence to support the interpretability of the primary clinical evidence (e.g., to demonstrate that the study population for an investigation conducted outside the United States (OUS) is representative of the US population, or to provide context for an adverse event observed in the clinical study);
- To generate evidence to support a petition for reclassification of a medical device under section 513(e) or (f)(3) of the FD&C Act;
In addition to the examples above, governments worldwide utilize RWD as important reference information when considering pharmaceutical and insurance systems and industrial policies.
In Europe and the United States, guidelines have also been established for the utilization of RWD in drug development. RWD are recognized as one of the comparators that confirm the efficacy and safety of drugs1)2). In the case of rare diseases, there is some difficulty confirming efficacy and safety in clinical trials of medicines because there are fewer patients. Therefore, RWD are a crucial source of information for understanding symptoms. However, when comparing the conclusion of clinical trials with RWD, the data must be the same quality as clinical trials4). As a result, further database enhancement for recording the treatment process, such as Patient Registries, is needed.
Protecting personal information is crucial when using RWD, but overly strict regulations can hinder implementation. Japan faces a difficult situation in using RWD/RWE compared to Europe and the United States5). For example, 90 cases of RWE utilization have been published and used by the people as a collection of case studies in the United States. By contrast, RWE are not fully utilized in Japan.
However, in addition to comprehensive databases such as “NDB (National Database)” provided by the Ministry of Health, Labour and Welfare of Japan and “MID-NET” managed and operated by the Pharmaceuticals and Medical Devices Agency in Japan, various databases such as “Rare Disease Data Registry of Japan” and those specialized for each disease have recently been developed in Japan. In addition, the “Japan National Medical Information Platform” is under development*1 to consolidate and integrate many RWD in Japan, which is expected to lead to more active utilization of RWD.
*1 : As of Aug 27, 2024
Real-World Data comprises a patient’s daily medical records and a single questionnaire. Bringing together data will lead to further improvements in the quality of medical care and the development of society.

Reference materials
- U.S. Food & Drugs, Considerations for the Use of Real-World Data and Real-World Evidence to Support Regulatory Decision-Making for Drug.
(https:// www.fda.gov/media/171667/download)(Accessed Aug 5, 2024) - European Medical Agency, Real-world evidence provided by EMA,
(https://www.ema.europa.eu/en/documents/other/guide-real-world-evidence- provided-ema-support-regulatory-decision-making_en.pdf) (Accessed Aug 5, 2024) - U.S. Food & Drugs, Draft: Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices,
(https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-use-real-world-evidence-support-regulatory-decision-making-medical- devices) (Accessed Aug 5, 2024) - Act on Quality, Efficacy and Safety Assurance of Pharmaceuticals and Medical Devices,
(https://www.mhlw.go.jp/web/t_doc?dataId=81004000&dataType=0&pageNo=1) (Accessed Aug 27, 2024) - National Institute of Biomedical Innovation Website, Survey of Real World Data (RWD) Applications in Medical Device Development,
(https://www.amed.go.jp/content/000128593.pdf) (Accessed Aug 5, 2024) - U.S. Food & Drugs, Examples of Real-World Evidence Used in Medical Device Regulatory Decisions,
(https://www.fda.gov/media/146258/download) (Accessed Aug 27, 2024) - Ministry of Health, Labour and Welfare of Japan, Meeting on the promotion of medical DX (SEPT 8, 2023),
(https://www.mhlw.go.jp/content/10808000/001144379.pdf) (Accessed Aug 27, 2024)

