Activities Report / InformationREPORT & INFORMATION
2024.06.03
Useful information
- #New drugs development
- #Clinical trial for regulatory submission
- #Clinical trial
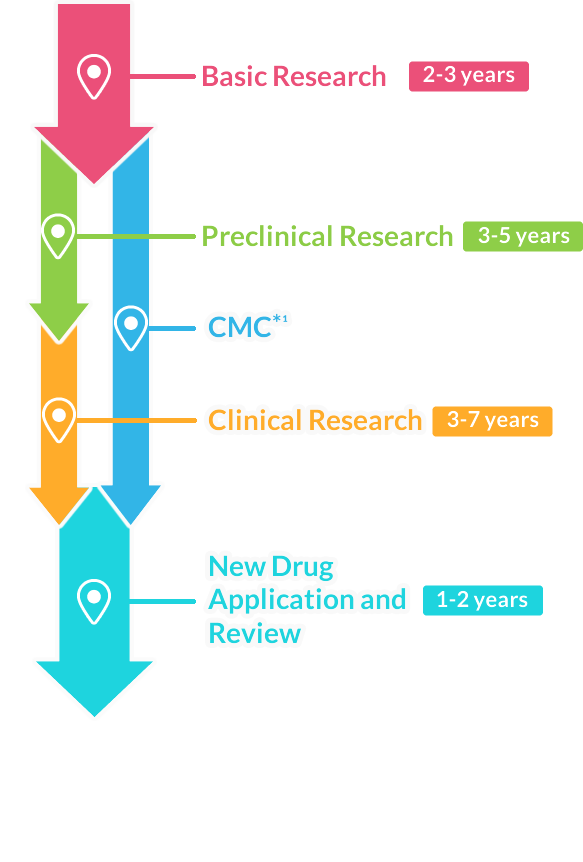
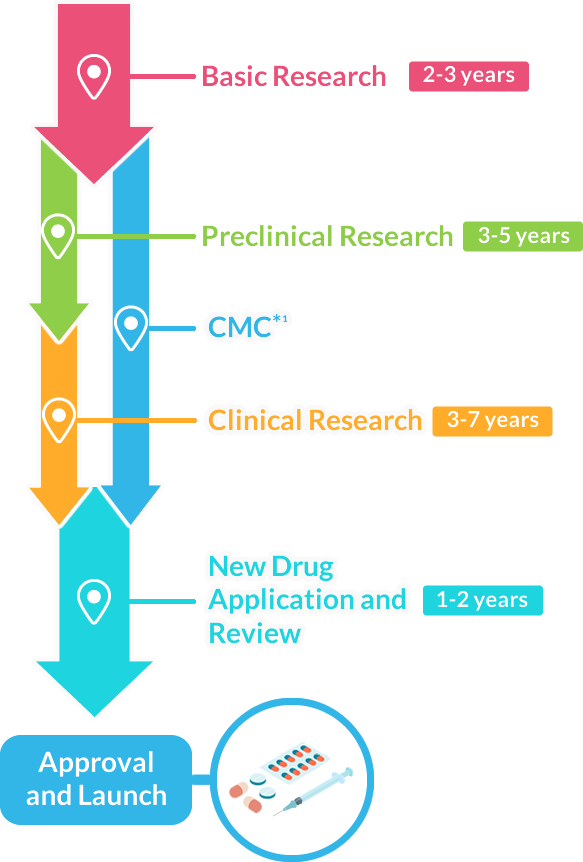
The Journey of New Drug Development
■ The following article is about the process of new drug development in Japan.
Have you ever thought about the medicine you take when you get sick or feel unwell,
how it has been discovered and how long it takes time to be developed?
Overall, it may take about 9-17 years
and is estimated to cost tens of billions of yen
to develop a single “new drug” in Japan.
*1 Chemistry, Manufacturing and Control research
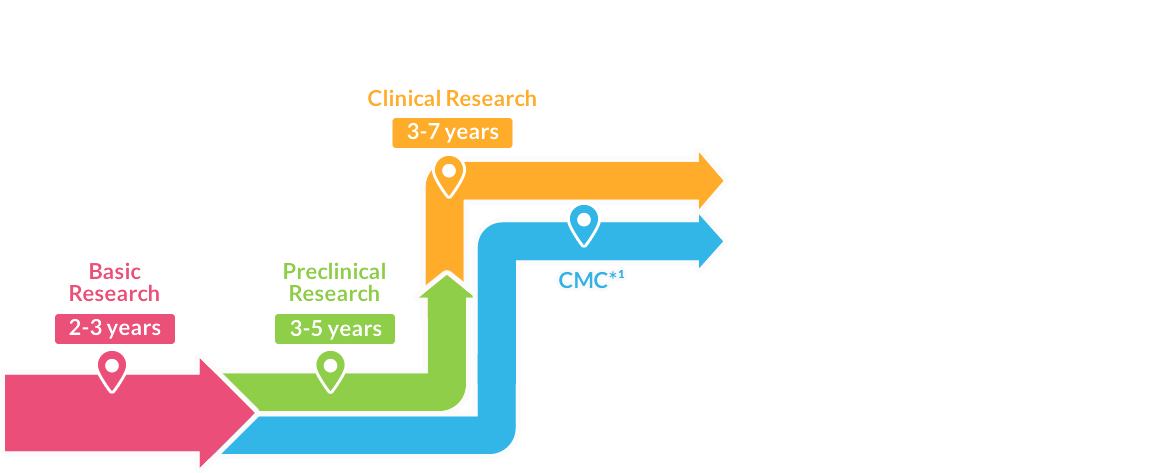
Basic Research 2-3 years
Drug development begins with researching the symptoms and suffering experienced by patients, then discovering the drug’s basis (substances/components) and conducting studies to chemically produce it. The drug candidate is found by extracting from plants and microorganisms or chemically synthesizing substances. Researchers spend a long time experimenting and searching for potential new drugs.

Preclinical Research
(in animal/cultured cells)3-5 years
This is to research the efficacy and safety of a new drug candidate using animals and cultured cell subjects.

Clinical Research (in humans)3-7 years
This research is to confirm the efficacy in humans for a new drug candidate (investigational drug) that has passed the necessary preclinical trials.

-
Phase
ⅠConfirmation of safety, such as side effects, in a small number of healthy adult volunteers
-
Phase
ⅡConfirmation of effective, safe dosage and administration method in a small number of consenting patients
-
Phase
ⅢConfirmation of the efficacy and safety in a large number of consenting patients
CMC
The process conducts quality evaluation, stability testing, and development of manufacturing methods for a drug candidate.

To ensure the safety, efficacy, and stable high-quality manufacturing of these candidates for human use, it is necessary to follow a series of processes as outlined below.
- Researching
technologies for the stable production of
active
pharmaceutical ingredients - Considering pharmaceutical formulation design (appearance, taste, size) for patients to take the product
- Quality assurance and
Quality control
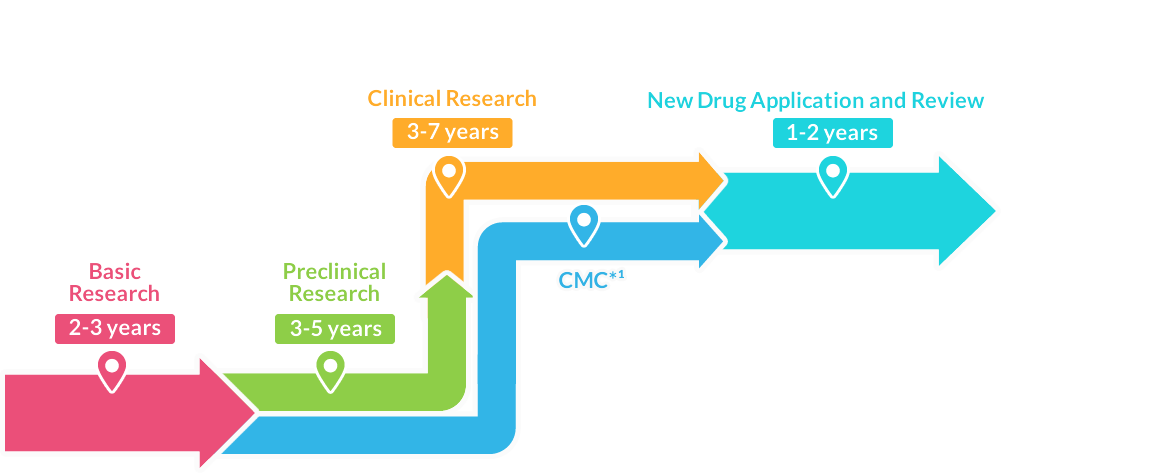
New Drug Application and Review1-2 years
After confirming efficacy, safety, and quality pass through clinical trials, an application is submitted to the Ministry of Health, Labor and Welfare for manufacturing and marketing approval.
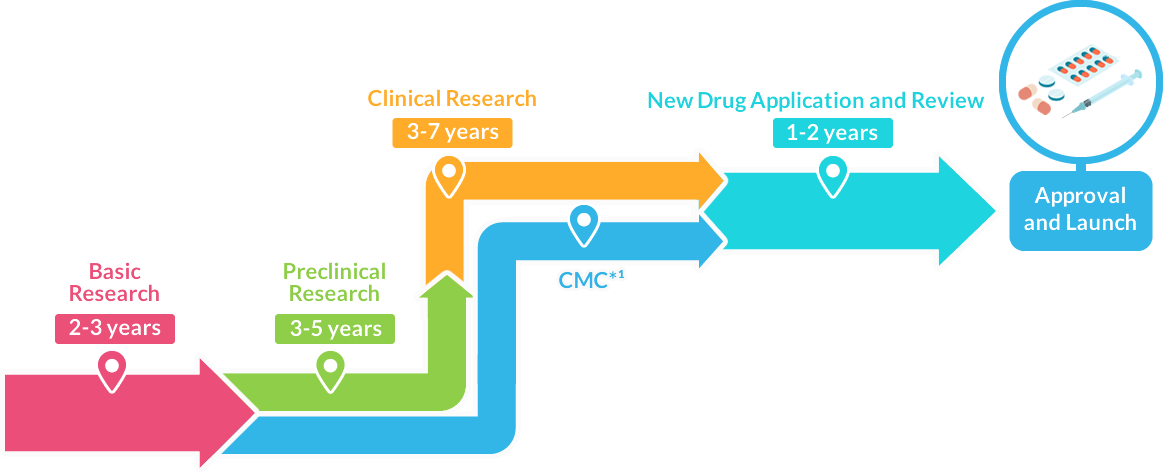
After receiving approval through the evaluation by the Pharmaceuticals and Medical Devices Agency (PMDA), the drug can be manufactured and launched as a new drug.
Approval and Launch
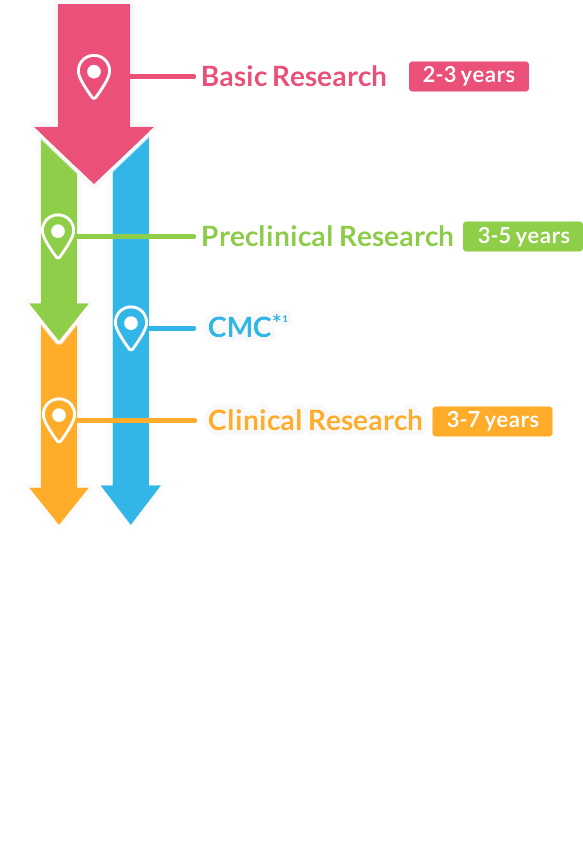
- Preclinical & Clinical Research
- CMC
Basic Research 2-3 years
Drug development begins with researching the symptoms and suffering experienced by patients, then discovering the drug’s basis (substances/components) and conducting studies to chemically produce it. The drug candidate is found by extracting from plants and microorganisms or chemically synthesizing substances. Researchers spend a long time experimenting and searching for potential new drugs.

Preclinical Research
(in animal/cultured cells)3-5 years
This is to research the efficacy and safety of a new drug candidate using animals and cultured cell subjects.

Clinical Research (in humans)3-7 years
This research is to confirm the efficacy in humans for a new drug candidate (investigational drug) that has passed the necessary preclinical trials.

-
Phase
ⅠConfirmation of safety, such as side effects, in a small number of healthy adult volunteers
-
Phase
ⅡConfirmation of effective, safe dosage and administration method in a small number of consenting patients
-
Phase
ⅢConfirmation of the efficacy and safety in a large number of consenting patients
New Drug Application and Review1-2 years
After confirming efficacy, safety, and quality pass through clinical trials, an application is submitted to the Ministry of Health, Labor and Welfare for manufacturing and marketing approval.
After receiving approval through the evaluation by the Pharmaceuticals and Medical Devices Agency (PMDA), the drug can be manufactured and launched as a new drug.

Approval and Launch

- Preclinical & Clinical Research
- CMC
Reference materials
- MHLW. 7th Expert Panel on Comprehensive Measures to Achieve a Rapid and Stable Supply of Pharmaceuticals
(https://www.mhlw.go.jp/content/10807000/001036959.pdf) (Accessed Mar 28, 2024) - JPMA. Textbook of Pharmaceutical Industry 2022-2023(https://www.jpma.or.jp/news_room/issue/textbook/index.html) (Accessed Dec 11, 2023)
- National Center for Child Health and Development. What is Clinical Research? (https://www.ncchd.go.jp/scholar/clinical/chiken/patient/about.html)
(Accessed Nov 22, 2023)