Activities Report / InformationREPORT & INFORMATION
2024.11.01
Medical and disease
information
- #Home-based Clinical Trial
- #Virtual Clinical Trial
- #New drugs development
Current Status and Future Prospects of Decentralized Clinical Trials (DCTs)
In recent years, advances in medical technology have accelerated the development of new drugs. Furthermore, the concept of Patient Centricity* has become widespread in drug development, with pharmaceutical companies increasingly incorporating patient feedback into drug development and clinical trial design. This creates an environment that makes it easier for patients to participate in clinical trials.
However, developing drugs for rare diseases presents various challenges, such as a shortage of specialist physicians and the distance and time constraints between the patient’s home and the clinical trial site. This sometimes leads to patients abandoning participation in clinical trials, creating a significant disparity in access to clinical trials across different regions.
To address this issue, “Decentralized Clinical Trials (DCTs),” which include home-based clinical trials, are on the rise.
This article explains DCTs, a new approach that will increase the chances of more patients participating in clinical trials in the future.
*Patient Centricity: A concept meaning “patient-centered.” It suggests that the three parties surrounding the patient, the medical institution, the regulatory authority, and the pharmaceutical company, should “always prioritize the patient, focus on the patient, and ultimately respect the patient’s judgment to the greatest extent possible.”
Table of Contents
1. What are Decentralized Clinical Trials?
Decentralized Clinical Trials (DCTs) are a new approach in which patients can participate in clinical trials from home for the development of new drugs and treatment methods.
Conventional clinical trials require frequent visits to the hospital. In contrast, DCTs involve doctors and nurses visiting patients at home or utilizing online medical services, significantly reducing the need for hospital visits.
DCTs could be a new option, especially for patients with rare diseases or those who live far away and find it difficult to visit the hospital.
Additionally, participating in clinical trials at home may reduce the mental burden on patients.
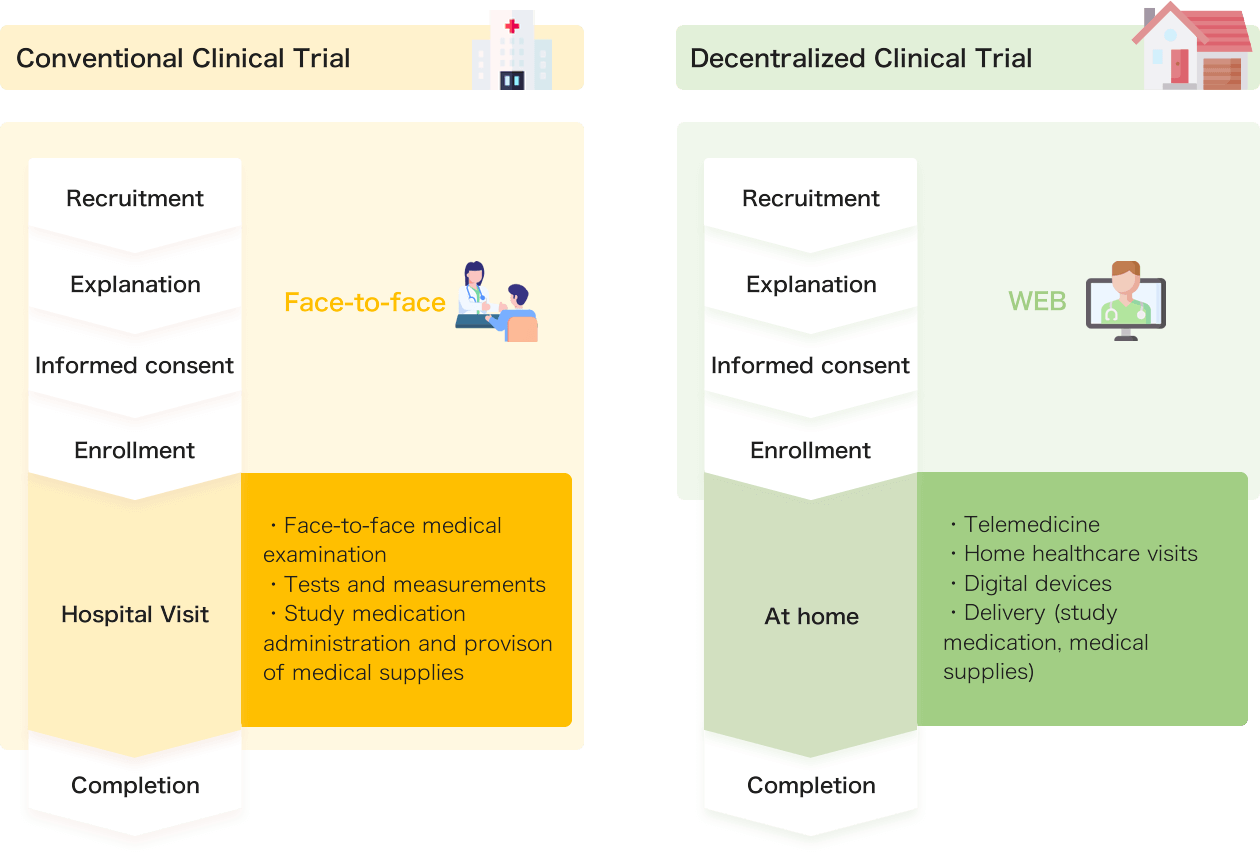
Reference: Japan Pharmaceutical Manufacturers Association. Study on the introduction and use of clinical trial methods that do not depend on visits to medical institutions.
(https://www.jpma.or.jp/information/evaluation/results/allotment/lofurc0000005jr6-att/tf3-cdt_00.pdf) (Accessed Sep 15, 2024)
2. Current Status of Decentralized Clinical Trials (DCTs) in Japan
In Japan, a gradually increasing number of pharmaceutical companies are conducting clinical trials that incorporate eConsent (a tool that uses images, audio, video, and other tools to enable effective explanation and consent), telemedicine, and direct delivery of study medications to the participant’s home.
According to a survey by the Drug Evaluation Committee of the Japan Pharmaceutical Manufacturers Association, as of 2020, no companies had experience with clinical trials using telemedicine. But in May 2024, five companies had implemented such trials, and seven were planning to do so.
Case Studies
- The Name of the medical institution
- Case
- National Cancer Center Japan
(2023) - Decentralized Clinical Trial:
Patients in remote areas who had difficulty visiting National Cancer Center Japan for the two investigator-initiated clinical trials for rare cancers can now participate in the clinical trials through nearby medical institutions due to the incorporation of the online clinical trial system. Not only will this dramatically improve access to clinical trials for patients living in rural areas, but it is also expected to accelerate the speed of enrolling the participants, resulting in earlier completion of clinical trials and lower overall clinical trial costs. - Aichi Cancer Center
(2022) - Remote Clinical Trial via Telemedicine in Collaboration with Family Hospital:
Clinical trials that were conventionally conducted in person were conducted via telemedicine. Telemedicine was made possible from the first visit by providing medical information in advance from the family hospital. By combining telemedicine with collaboration with the family hospital and home delivery of study medications, Aichi Cancer Center has realized a fully remote clinical trial in which patients participate in a new drug trial without ever visiting to the sites.
Reference: “Current Status and Potential of Digital Transformation (DX) in Clinical Development,” Drug Evaluation Committee, Japan Pharmaceutical Manufacturers Association.
(https://www.jpma.or.jp/information/evaluation/results/allotment/tcjmdm0000001ecw-att/CL_202405_TF1_DX.pdf) (Accessed Sep 15, 2024).
3. Challenges of Decentralized Clinical Trials (DCTs) in Japan
The following issues are considered to be challenges to the widespread adoption of DCTs in Japan.
- Issues
- Issues related to telemedicine
-
- Less information compared to in-person treatment.
- Insufficient social infrastructure for telemedicine itself.
- Insufficient clear standards for the eConsent process.
- Decrease in patient engagement when the frequency of in-person treatment drops due to the replacement of in-person visits with telemedicine in DCTs.
- Issues related to conducting clinical trials
-
- Training and securing nurses to perform clinical trial duties.
- Handling samples when blood is taken at the participant’s home.
- Drug stability, temperature control, costs, and handling of personal information during direct delivery of study medications and related materials to participant’s home.
- Issues related to Data management
-
- Ensuring regulatory compliance for various data types, such as patient-reported outcomes (ePRO), wearable device data, and remotely collected clinical data.
- Development of new indicators consistent with the Virtual Clinical Trials and patient centricity.
- Ensuring data quality and reliability.
- Ensuring the handling of personal and safety information associated with the data.
Reference: Japan Pharmaceutical Manufacturers Association, “Study on the introduction and use of clinical trial methods that do not depend on visits to medical institutions.” (https://www.jpma.or.jp/information/evaluation/results/allotment/lofurc0000005jr6-att/tf3-cdt_00.pdf) (Accessed Sep 15, 2024)
In addition, although this issue is not limited to DCTs, media such as websites are currently underutilized in helping patients recognize and proactively participate in clinical trials.
4. Trends in Overseas Decentralized Clinical Trials (DCTs)
In the United States and Europe, DCTs were introduced earlier than in Japan and have a history of over 20 years. In particular, clinical trials utilizing wearable devices have been conducted since the 2010s and have evolved with technology and new methods.
In the United States, for example, DCTs have been incorporated in various stages of development and therapeutic areas. In particular, some pharmaceutical company-sponsored trials are fully Virtual Clinical Trials with no visits to medical institutions.
These trials are designed to reduce distance and time constraints on patient participation by online recruitment, eConsent, and telemedicine.
In addition, various data collection methods have been implemented, including electronic patient diaries (eDiary), wearable devices, and remote clinical assessments. Communication strategies are implemented to ensure patient safety and promote active involvement in clinical trials.
Patients have given positive feedback, citing flexibility and convenience as the main advantages.
5. Future Prospects for Decentralized Clinical Trials (DCTs) in Japan
DCTs in Japan are still developing. But a support system is being established through the efforts of the government, medical institutions, pharmaceutical companies, and others to address patients’ concerns and questions and enable them to participate in clinical trials with peace of mind.
There is hope that new opinions will be offered through DCTs for many patients, including those with rare diseases.
Reference materials
- Japan Pharmaceutical Manufacturers Association. Study on the introduction and use of clinical trial methods that do not depend on visits to medical institutions.
(https://www.jpma.or.jp/information/evaluation/results/allotment/lofurc0000005jr6-att/tf3-cdt_00.pdf) (Accessed Sep 15, 2024) - National Cancer Center. Start of decentralized clinical trials for rare cancers: Enabling patients to participate in physician-initiated clinical trials conducted by Natural Cancer Center Hospital without leaving the region. June 27, 2023.
(https://www.ncc.go.jp/jp/information/pr_release/2023/0627_1/index.html) (Accessed Sep 15, 2024) - Heiikukai Medical Corporation. About Clinical Trials and Clinical Research.
(https://www.heiikukai.com/clinical-trial/) (Accessed Sep 15, 2024) - Japan Pharmaceutical Manufacturers Association, Drug Evaluation Committee. Current Status and Potential of Digital Transformation (DX) in Clinical Development.
(https://www.jpma.or.jp/information/evaluation/results/allotment/tcjmdm0000001ecw-att/CL_202405_TF1_DX.pdf) (Accessed Sep 15, 2024) - Japan Pharmaceutical Manufacturers Association, Drug Evaluation Committee. Drug Development with the Voice of Patients -Patient Centricity by Pharmaceutical Companies-. 2018.
(https://www.jpma.or.jp/information/evaluation/results/allotment/lofurc0000005m95-att/patient_centricity.pdf) (Accessed Sep 15, 2024)

