Activities Report / InformationREPORT & INFORMATION
2025.04.01
Useful information
- #Clinical trial for regulatory submission
- #Clinical trial
Introduction to Clinical Trial Database
■ This article is intended for residents in Japan.
This information is designed to enhance your understanding of clinical trials.
If you need medical advice, including participation in a clinical trial, please consult your physician.
- Regarding Clinical Trial Information
-
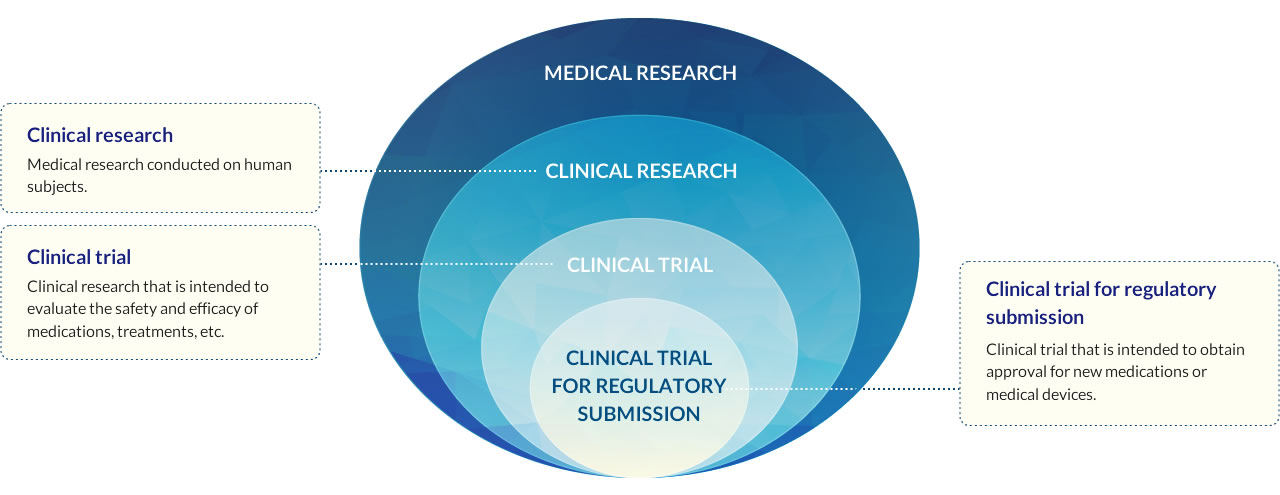
Clinical trials are studies conducted on human subjects for the purpose of evaluating the safety and efficacy of medications and medical devices. Among them, research conducted for the purpose of obtaining manufacturing and marketing approval is a clinical trial for regulatory submission.
Many clinical trials are conducted throughout the world to address diseases and their symptoms. Websites are available for the public, health care professionals, and researchers to search for information on the trials that interest them.
Patients often search for clinical trial information, and in the interest of transparency, clinical trials are required to be registered in clinical trial databases. Here, we will introduce some of the most representative websites and how to use them (please refer to the manual of each site for details on their use).We introduce not only the public websites in Japan but also the websites containing publicly available information on clinical trials in the US and Europe, global trials for which Japan is likely to be among the countries participating. In addition, there are many other websites that have been set up by various organizations. Please refer to these websites to help you find a site that is easy to use and is specialized in the area of your interest.
The sites mentioned in this article will provide you with mainly the following information.
- Name of medication* used and its administration
- Whether the trial is currently recruiting subjects
- Conditions for participation (target age, symptoms, etc.)
- Participating medical sites
- Responsible trial personnel and contact information
*Medication: Includes study medications
- Clinical Trial Information Databases
-
● jRCT (Japan Registry of Clinical Trials): A system for disclosing clinical research protocols and synopses
This is a public site where you can find information on all clinical trials conducted in Japan (completed trials are included). However, the timing of when trial information is updated varies depending on the company and researcher.
To search for information on medical research in general, the Clinical Research Information Portal Site, another public website, collects and publishes a wide range of information.Go to external site
Please note that the linked websites are not operated by MEDIPAL Group.
To the external sitesHow to use it:
-
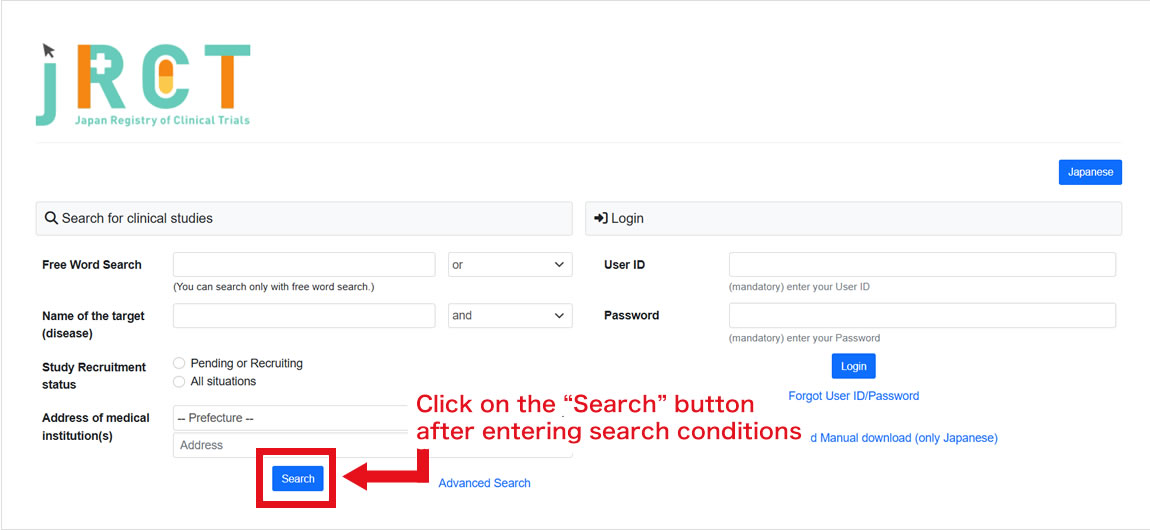
1Open the Japan Registry of Clinical Trials (external site), and you will see a search field in the top of the page. No login is required.
Go to external site
Please note that the linked websites are not operated by MEDIPAL Group.
To the external sites -
2Set your preferred search criteria and click on the “Search” button. When the desired trial is displayed, click the “View” button to see the details of the trial. You can also perform detailed searches.
A system for disclosing clinical research protocols and synopsesGo to external site
Please note that the linked websites are not operated by MEDIPAL Group.
To the external sites● ClinicalTrials.gov
This is a database maintained by the US National Institutes of Health (NIH) that registers current and previous clinical trials. Some trials conducted in Japan are also registered.
How to use it:
-
1Open ClinicalTrials.gov (external site).
Go to external site
Please note that the linked websites are not operated by MEDIPAL Group.
To the external sites -
2A screen for entering search criteria will open. Enter the search criteria of your choice, including “Condition/disease”, “Other terms”, “Intervention/treatment”, or “Location.”
ClinicalTrials.govGo to external site
Please note that the linked websites are not operated by MEDIPAL Group.
To the external sitesGo to external site
Please note that the linked websites are not operated by MEDIPAL Group.
To the external sites● Clinical Trials Information System (CTIS)
The database is managed by the European Medicines Agency (EMA) for the registry of clinical trials conducted in the EU and EEA regions.
All ongoing clinical trials in Europe are registered in the CTIS.*The data transition period from the EU Clinical Trial Register (CTR) ended on January 30, 2025.
How to use it:
-
1Open the “Clinical Trials Information System (CTIS)”(external site).
Go to external site
Please note that the linked websites are not operated by MEDIPAL Group.
To the external sites -
2Select “Search for trials” in the upper menu, or select “Search clinical trials” in the middle of the text.
-
3After the search box appears, enter the words you want to search into the search box, such as contain all these terms, contain any of these terms, do not contain any of these terms, or several detail conditions.
● Other: Pharmaceuticals and Medical Devices Agency (PMDA): Information on main clinical trials conducted from humanitarian perspective (expanded clinical trials)
Pharmaceuticals and Medical Devices Agency (PMDA)Go to external site
Please note that the linked websites are not operated by MEDIPAL Group.
To the external sites -
Disclaimer
- ● The contents of this information, the screens cited, and the websites introduced are current as of the date of preparation. Please note that the information and URL links may have changed.
- ● This information and the publicly available information in the websites presented within are not intended to provide medical advice. If you have any medically related questions, concerns, or other inquiries, including participation in a clinical trial, be sure to consult with your physician.
- ● This information is not intended to encourage participation in a corporate-sponsored clinical trial.
Reference materials
- jRCT (Japan Registry of Clinical Trials) website (https://jrct.mhlw.go.jp/) (as of Mar 25, 2025)
(2024.01.22 release/2025.04.01 update)

